Have you ever wondered how fireworks get their bright and vibrant colors? Well, it’s all chemistry, a chemical reaction created by combining an oxidizer, some kind of fuel, and metal salts which when burned, let off different wavelengths of light or colors.
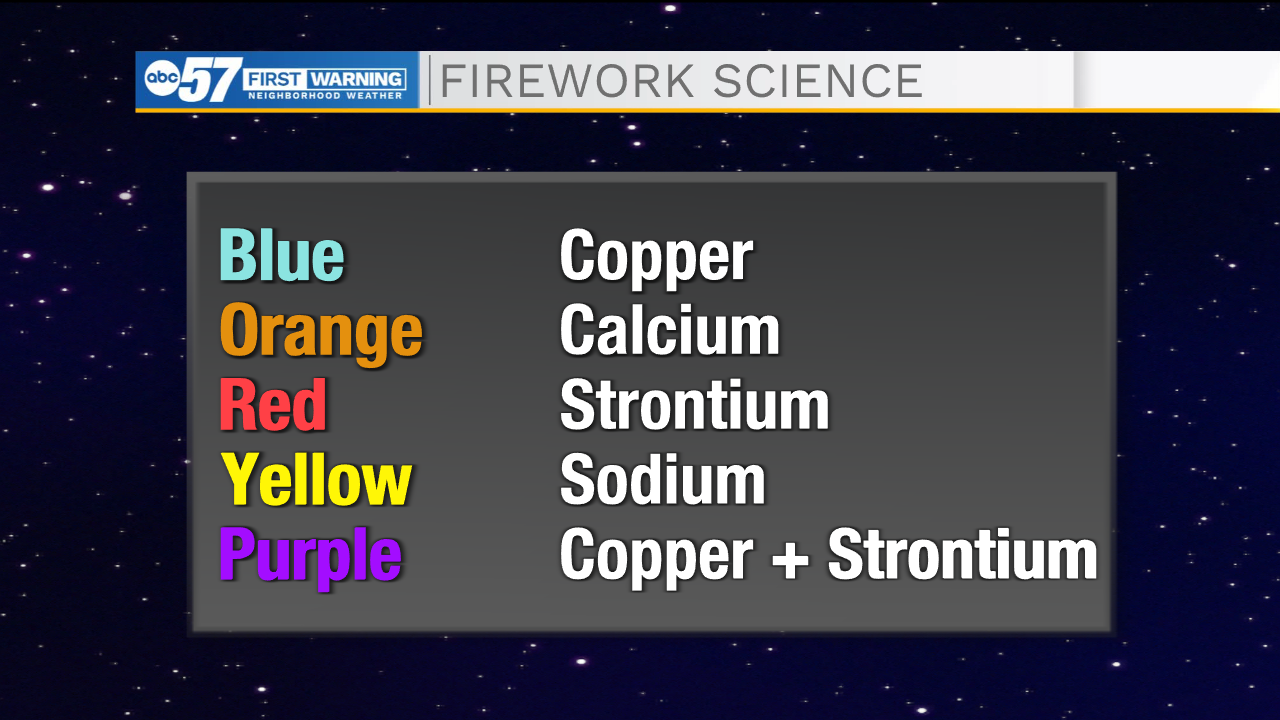
Blue fireworks are made using copper salts and are the hardest to produce pyrotechnically speaking due to a balance of heat and chemicals involved being harder to achieve.
Orange fireworks are produced by burning calcium salts, a material that many of us know to be important for bone health. The bright red fireworks are created using strontium salts, a compound used in flare guns. Yellow fireworks are possible thanks to sodium salts, which are also used in the production of many household items such as cleaning products or fertilizer.
Some firework colors are created using a combination of metal salts, such as purple fireworks which are produced using the ingredients for red and blue fireworks - copper and strontium salts. If the ingredients that make up the firework are impure at all, the color can be altered or too much smoke can be produced to see the firework well.
Some colors need to be created using salts that are chemically unstable at room temperature, and must be combined with another, more stable compound that will be burned off in the lighting of the firework. Some salts are instead unstable at too high of temperatures, so a balance has to be found between the firework burning hot enough to be seen clearly, but also not too hot that the color gets washed out.
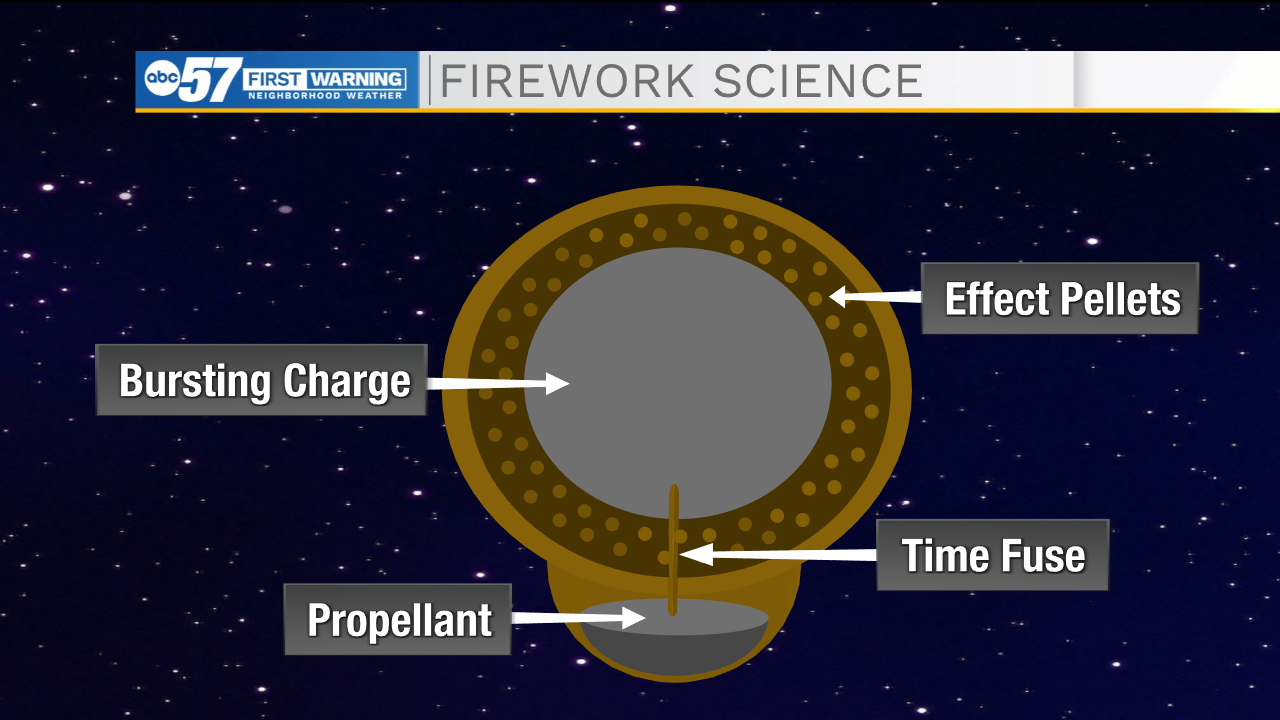
Fireworks start out as small capsules called aerial shells. These shells hold all the ingredients needed for a firework – all it needs is a spark.
First, the shell is placed in a tube where a black powder propellent at the bottom of the shell is lit using a fuse to launch into the air. A time fuse connects the propellent to a bursting charge made up of more black powder in the center of the shell, delaying the time that the bursting charge and therefore, firework, go off. This is what allows the firework to explode high up in the air rather than close to the ground.
The bursting charge is surrounded by small marble-sized pellets that hold the metal salts and release the color we see in the night sky. These pellets can be arranged in different orders to create different effects and shapes.
Lighting off fireworks can be a great way to celebrate the 4th of July, but it can also be dangerous. It can be easy to get hurt when handling fireworks at home, especially if the fireworks you’re using aren’t very good quality.
Aerial shells that hold all the ingredients for fireworks are filled with gunpowder, so a small – or big – explosion happens every time one is set off. In 2021, 74% of firework related injuries happened during the weeks before and after the 4th of July holiday.
If you’d like to see some fireworks today but want to leave the pyrotechnics to the experts, you can head over to Four Winds Field tonight. The South Bend Cubs will be taking on the Wisconsin Timber Rattlers at 7:05pm, and fireworks will be lit off after the game ends.
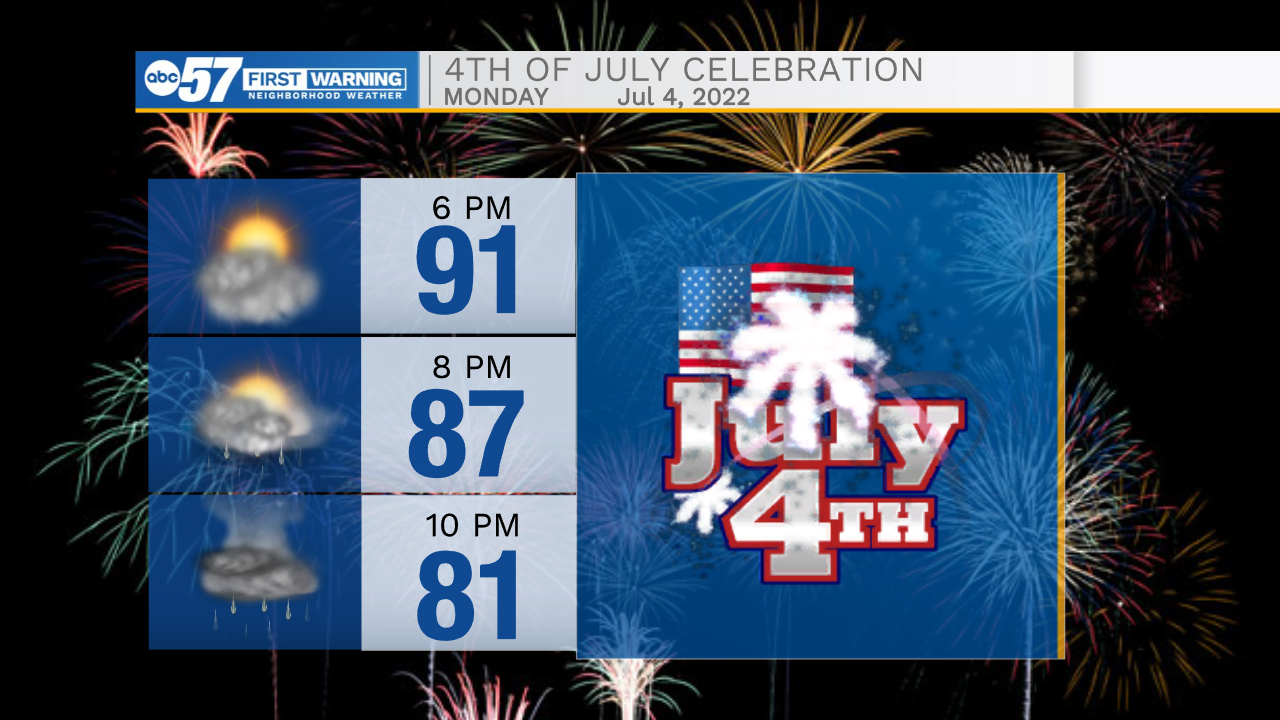
Temperatures will be hot as the game starts, and will remain warm throughout the evening. There is a small chance for rain showers while the game is going on, so have your umbrella on standby, and make sure to keep an eye on the radar today.